- connect@cliorbit.in
- 8767307223
Leading Pharmacovigilance Services in India | Cliorbit
Transform your drug safety operations with India's premier pharmacovigilance service provider
Optimized the collection, evaluation, and reporting of adverse events to meet Indian and international regulatory standards.
Cutting‑edge statistical techniques and AI‑driven tools for prompt detection of possible safety signals in clinical and post‑marketing data.
Specialized advice on CDSCO filings, FDA compliance, and worldwide pharmacovigilance regulatory requirements.
ISO‑certified workflows with a 99.8% data accuracy rate and thorough audit‑trail documentation.

Who we are
Leading Pharmacovigilance Services in India
Why Choose Cliorbit for Pharmacovigilance Services in India?
work we do
Comprehensive Drug Safety Solutions
Adverse Event Reporting (AER) and ICSR Processing
- Signal Detection and Risk Management Planning
- Post-Market Surveillance and Drug Safety Monitoring
- Regulatory Compliance Support (CDSCO, FDA, ICH)
- Literature Review and Medical Writing Services
- Pharmacovigilance System Setup and Maintenance
Pan‑India Presence with Regional Insight**
Active in Mumbai, Bangalore, Hyderabad, Chennai, Delhi NCR, Pune, and Ahmedabad, we deliver customized pharmacovigilance support backed by a thorough grasp of local healthcare frameworks and reporting mandates.
**Adverse Event Management**
Optimized collection, evaluation, and reporting of adverse events, guaranteeing adherence to Indian and worldwide regulatory standards.
**Safety Signal Detection**
Cutting‑edge statistical techniques and AI‑driven tools for early spotting of possible safety signals in clinical and post‑market datasets.
**Regulatory Affairs Support**
Specialized assistance with CDSCO filings, FDA conformity, and global pharmacovigilance regulatory obligations.
**Quality Assurance Excellence**
ISO‑certified workflows delivering 99.8 % data accuracy and full audit‑trail documentation.
Project Done
Country Branch
Client Rating
Years Experience
HOW TO START & CLIORBIT pROCESS.
Getting Started with Cliorbit Pharmacovigilance Services
Start your pharmacovigilance journey with a thorough evaluation of your drug safety needs, regulatory duties, and operational goals. Our specialists will create tailored solutions that fit your exact requirements.
Smooth shift to Cliorbit’s pharmacovigilance services via comprehensive project planning, system installation, workforce training, and incremental rollout, ensuring minimal disruption to existing operations.
Adopt a long‑term partnership model with a dedicated account manager, periodic performance reviews, ongoing improvement initiatives, and proactive adjustments to shifting regulatory requirements.

What we offer
Complete Pharmacovigilance Services Portfolio
Why Choose Us
Industries We Serve Across India
Providing extensive pharmacovigilance support to leading Indian pharmaceutical manufacturers and multinational firms, covering generic medicines, branded formulations, and complex molecules across all therapeutic categories.
Offering dedicated safety solutions for contract research organizations conducting clinical trials in India, covering Phase I‑IV studies, bioequivalence assessments, and post‑marketing surveillance, fully adhering to regulatory standards.
Specialized pharmacovigilance assistance for biotech firms creating cutting‑edge treatments—biosimilars, monoclonal antibodies, vaccines, and gene therapies—that demand advanced safety monitoring protocols.
Tailored assistance for worldwide pharmaceutical companies launching operations in India, covering regulatory compliance advice, establishment of local safety databases, and support for CDSCO registration.
- Professional Medical Assessment and Evaluation
- ICSR Generation and Quality Control
- Adverse Event Reporting Process Excellence
- Regulatory Timeline Management
- Quality Assurance and Data Validation
- Standardized Case Processing Workflow
What is Pharmacovigilance?
Pharmacovigilance is the discipline and set of activities focused on the identification, evaluation, comprehension, and prevention of adverse drug reactions or any other medication-related issues. In India, pharmacovigilance goes beyond simple adverse event reporting to include thorough post-marketing surveillance, proactive risk-management strategies, and ongoing benefit-risk assessment throughout the product’s life cycle.
Why Pharmacovigilance Matters in India
- Varied Population: The country’s genetic heterogeneity, differing nutritional habits, and diverse disease patterns produce unique adverse-event signatures that demand tailored monitoring methods.
- Healthcare Infrastructure: Disparities in care quality between urban centers and rural regions influence the detection and reporting of safety events.
- Widespread Polypharmacy: Extensive use of multiple concurrent medications adds complexity to causality analysis and drug-interaction surveillance.
- Regulatory Progress: The CDSCO’s increasingly stringent pharmacovigilance rules align with global standards while meeting domestic market requirements.
Business Benefits of Pharmacovigilance Excellence
- Up to 85% fewer regulatory compliance breaches and related fines.
- A 40-60% reduction in operating expenses via streamlined processes and strategic outsourcing.
- Approximately 75% gains in the precision of product labeling and the effectiveness of risk communication.
- Roughly 90% faster approval of regulatory submissions thanks to high-quality safety documentation.
CDSCO Regulatory Framework and Compliance Obligations
Central Drugs Standard Control Organization (CDSCO) Overview
CDSCO is India’s national regulatory body managing pharmacovigilance with guidelines aligned to WHO and ICH standards while addressing India-specific requirements.Required Pharmacovigilance System Components
- Qualified Person Responsible for Pharmacovigilance (QPRP): An appointed individual with medical/pharmaceutical credentials, responsible for sustaining pharmacovigilance and available for CDSCO queries.
- Pharmacovigilance System Master File (PSMF): Comprehensive documentation of system structures, SOPs, and procedures, updated as changes occur.
- Safety Database and Case Management System: Validated IT system for adverse event data collection meeting integrity and audit requirements.
- Adverse-Event Reporting Obligations: Serious events reported within 15 days for fatal/life-threatening, 90 days for others; includes annual safety summaries and periodic reports.
CDSCO Inspection Readiness
- System adequacy and procedure compliance
- Quality and timeliness of case documentation
- Staff competence and training records
- Data integrity and audit trails
- Effectiveness of corrective and preventive actions
Frequent Compliance Gaps and Their Impact
- Poor documentation causing warning letters and remediation
- Late reporting leading to registration delays
- System weaknesses requiring upgrades and re-validation
- Training deficiencies triggering extra oversight
: Building vs. Outsourcing – Strategic Decision Framework
Developing an In-House Pharmacovigilance Function
Benefits of an Internal System
- Full control over processes and quality
- Deep product and therapeutic knowledge
- Integration with clinical development and regulatory affairs
- Long-term cost efficiencies with large portfolios
Challenges of Internal Implementation
- High upfront costs ($500K – $2M)
- Ongoing expenses for staff and maintenance
- Need for regulatory expertise
- Scaling difficulties with growth
Outsourcing to Specialized PV Service Providers
Strategic Advantages
- Access to niche regulatory and PV expertise
- Lower, predictable costs with 40-60% savings
- Scalable operations without internal resource limits
- Risk reduction from provider experience
Critical Success Factors for Outsourcing
- Provider expertise, technology, and cultural fit
- Clear SLAs and performance measures
- Strong communication and escalation paths
- Continuous performance review and improvement
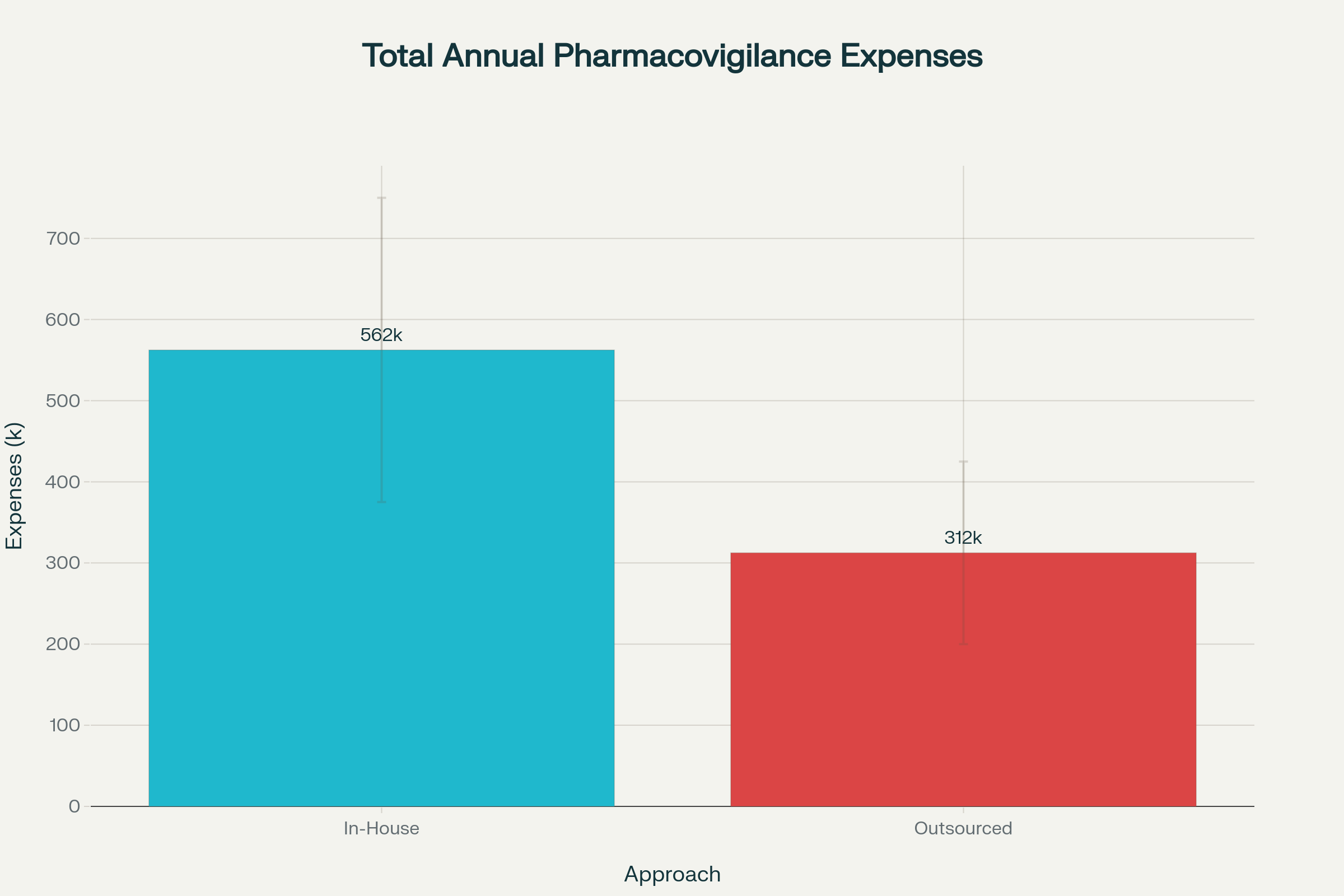
Financial Comparison: In-House vs. Outsourced
| Expense Category | In-House Annual Range (USD) | Outsourced Annual Range (USD) |
|---|---|---|
| Personnel (FTEs) | 150,000 – 300,000 | Included in fees |
| System licenses and upkeep | 100,000 – 200,000 | Included in fees |
| Infrastructure and overhead | 75,000 – 150,000 | Included in fees |
| Training and compliance | 50,000 – 100,000 | Included in fees |
| Total Range | 375,000 – 750,000 | 200,000 – 425,000 |
Technology Platform Selection & Implementation
Essential Technology Needs
Fundamental System Functions:
- Automated intake of cases from varied sources (clinicians, patients, publications)
- Medical coding linked to MedDRA and WHO Drug Dictionary
- Workflow control with role‑based permissions and approval steps
- Capabilities for regulatory filings (E2B, PSUR, annual reports)
- Tools for signal detection and risk management
- Audit‑trail and data‑integrity compliance
Top Pharmacovigilance Solutions:
- Oracle Argus Safety – industry‑standard offering full‑range features
- ArisGlobal LifeSphere – cloud‑native with sophisticated analytics
- Veeva Vault Safety – modern UI and strong integration options
- IQVIA VigiFlow – worldwide database supporting extensive regulatory submissions
Implementation Schedule & Approach:
- Months 1‑2: Gather requirements and configure the system
- Months 2‑3: Migrate data and conduct integration testing
- Months 3‑4: Conduct user training and validate processes
- Months 4‑5: Run parallel processing while monitoring performance
- Month 6 +: Switch to full‑scale operation and pursue ongoing optimization
Technology ROI Factors:
- Automation cuts case‑handling time by 60‑75 %
- Built‑in submission tools shorten regulatory timelines by roughly 40 %
- Advanced analytics boost signal‑detection accuracy up to 300 %
- Audit‑ready documentation trims inspection prep effort by 80 %
Quality Management & Operational Excellence
Quality Management System Structure
ISO 9001:2015 Alignment:
- Procedures for document control and management
- Risk‑based thinking coupled with continual improvement
- Emphasis on customer focus and stakeholder communication
- Systems for performance monitoring and management review
Good Pharmacovigilance Practice (GVP) Execution:
- Planning and assurance of quality processes
- Staff qualification and training programs
- Standard operating procedures and work instructions
- Change‑control and configuration‑management processes
- Corrective‑and‑preventive‑action (CAPA) mechanisms
Key Performance Indicators (KPIs)
Compliance Metrics:
- Adherence to regulatory timelines: ≥ 95 %
- Case completeness & quality: ≥ 98 %
- Closure of inspection findings: within 30 days
- Training completion: 100 %
Operational Efficiency Metrics:
- Case‑processing cycle time: target < 5 days
- Cost per processed case: benchmark for optimization
- System uptime/availability: ≥ 99.5 %
- Customer‑satisfaction rating: ≥ 90 %
Continuous Improvement Framework
- Periodic Performance Reviews – monthly analysis of metrics and trend spotting
- Process Optimization – quarterly workflow assessments and enhancement rollout
- Technology Upgrades – yearly system updates and capability extensions
- Best‑Practice Adoption – industry benchmarking and methodological refreshes
Risk Management & Compliance Assurance
All-Encompassing Risk Evaluation Framework
Regulatory Compliance Risks
- Penalties for CDSCO non-conformity and loss of market entry
- Global regulatory alignment and cross-border submission effects
- Inspection outcomes and necessary corrective actions
- Breaches of timelines and resulting business impact
Operational Risk Reduction
- Employee turnover and knowledge-retention tactics
- System outages and business-continuity planning
- Data security and confidentiality safeguards
- Dependence on third-party vendors and service-continuity guarantees
Strategic Risk Oversight
- Preserving market reputation and stakeholder trust
- Gaining a competitive edge via superior safety records
- Protecting investments with scalable, sustainable infrastructures
- Managing regulator relationships and top-notch communication
Insurance & Legal Aspects
- Professional indemnity for pharmacovigilance duties
- Data-privacy compliance (GDPR alignment)
- Risk sharing in outsourcing contracts
- Safeguarding intellectual property and confidentiality
– Vendor Selection & Partnership Management
Holistic Vendor Evaluation Criteria
Technical Capability Review
- Depth of regulatory know-how and compliance history
- Sophistication of tech platform and integration ease
- Medical/scientific expertise level
- Maturity of quality-management system and relevant certifications
Operational Excellence Review
- Ability to scale and manage capacity
- Geographic reach and time-zone coverage
- Language skills and cultural awareness
- Emergency response plans and escalation protocols
Financial Health & Viability
- Overall fiscal stability and long-term sustainability
- Competitive pricing and value proposition
- Contractual terms and SLA clarity
- Insurance coverage and liability safeguards
Due-Diligence Steps
- RFP: Define detailed specs and selection criteria
- Vendor Review: Conduct technical demos and verify capabilities
- Reference Checks: Collect client feedback and case-study proof
- Site Visits: Inspect facilities and meet the team
- Pilot Projects: Run limited-scope tests to gauge performance
- Contract Negotiation: Refine terms and allocate risks
Keys to Partnership Success
- Established communication channels and regular performance audits
- Joint problem-solving mindset with a culture of continuous improvement
- Building mutual respect and trust over time
- Aligning strategically and sharing success metrics
Implementation Roadmap & Change Management
Step-wise Deployment Plan
Stage 1: Foundations (Months 1-3)
- Choose vendor and finalize contracts
- Configure the system and perform initial setup
- Train the core team and build competencies
- Document processes and develop procedures
Stage 2: Transition (Months 3-6)
- Migrate data and integrate with existing systems
- Run parallel processing and verify quality
- Extend training to broader teams and enhance capabilities
- Monitor performance and fine-tune operations
Stage 3: Optimization (Months 6-12)
- Transfer full operational responsibility
- Deploy and leverage advanced functionalities
- Implement continuous-improvement initiatives
- Plan strategically for capacity growth
Change Management Guidelines
- Secure executive sponsorship and leadership buy-in
- Maintain clear communication and engage stakeholders
- Offer training and skill-development programs
- Track performance and provide feedback loops
- Celebrate achievements and recognize contributions
Success Indicators & Monitoring
- Sustain and improve regulatory compliance
- Achieve operational efficiency gains and cost savings
- Enhance quality while reducing errors
- Boost stakeholder satisfaction and confidence
Action Plan for Your Organization
- Review existing pharmacovigilance functions and compliance posture
- Set strategic goals and define success metrics
- Weigh build-versus-outsource options according to your unique requirements
- Choose qualified partners or launch internal capability development
- Deploy comprehensive systems with effective change-management practices
- Track performance and perpetually fine-tune operations
Prepared to Advance Your Pharmacovigilance Operations?
Reach out to Cliorbit for a full assessment of your pharmacovigilance needs and bespoke recommendations that ensure regulatory compliance, operational brilliance, and a competitive edge in India’s pharmaceutical sector.
Cliorbit blends clinical research proficiency with pharmacovigilance excellence to provide superior drug‑safety solutions customized for the Indian market.
Common Questions
Most Popular Questions
Pharmacovigilance is the discipline and associated activities focused on identifying, evaluating, comprehending, and avoiding adverse drug reactions or other medication‑related problems. In India, it is essential for protecting patient health, meeting the CDSCO’s regulatory standards, and bolstering the nation’s standing as a worldwide hub for pharmaceutical production.
The CDSCO mandates that pharma companies develop complete pharmacovigilance systems, which must include timely reporting of adverse events, appointing qualified pharmacovigilance officers, maintaining safety databases, submitting regular periodic safety update reports (PSURs), and executing risk‑management plans for their marketed products.
Although safety monitoring in clinical trials concentrates on adverse events under controlled study settings, pharmacovigilance involves thorough post‑marketing surveillance across varied patient groups, real‑world environments, and ongoing safety assessment throughout a product’s life cycle.
Serious adverse events are those that cause death, pose a threat to life, require an inpatient stay or extend an existing stay, lead to ongoing or major disability or incapacity, involve congenital anomalies or birth defects, or represent medically important incidents that need intervention to prevent serious consequences.
The CDSCO mandates that serious adverse events be reported within 15 days when they are fatal or life‑threatening, and within 90 days when they are non‑serious. For clinical trials, any serious, unexpected adverse reaction must be submitted as an expedited report within 7 days.
Causality assessment requires a systematic evaluation using recognized criteria such as the WHO‑UMC causality categories, taking into account factors like the temporal relationship, biological plausibility, de‑challenge/re‑challenge outcomes, concurrent medications, and alternative explanations for the observed adverse event.
A comprehensive adverse event report should contain patient data (age, sex, medical background), product specifics (name, dosage, indication, batch number), a detailed account of the adverse event (onset, outcome, seriousness), any concurrent medications, the patient’s medical history, and the reporter’s contact information.
Achieving CDSCO compliance involves setting up qualified pharmacovigilance systems, designating accountable individuals, keeping validated safety data repositories, adhering to good pharmacovigilance practices, performing periodic compliance audits, and staying abreast of evolving regulatory requirements and guidance documents.
CDSCO inspections assess the adequacy of pharmacovigilance systems, scrutinize case‑handling procedures, evaluate the quality and completeness of data, examine compliance with regulatory submissions, confirm training documentation, and review the implementation of SOPs and related documentation practices.
India’s pharmacovigilance rules closely follow ICH guidelines and WHO standards, while also incorporating distinctive CDSCO provisions. Global companies frequently adopt unified systems that simultaneously satisfy Indian, FDA, EMA, and other regulatory requirements.
Book an Appointment
Your trusted partner for comprehensive pharmacovigilance
9082731287
